Abstract
Introduction: Idecabtagene vicleucel (ide-cel), a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cell therapy, is approved by the US FDA for the treatment of adult patients (pts) with relapsed and refractory multiple myeloma (RRMM) after ≥ 4 prior lines of therapy, including an immunomodulatory agent, proteasome inhibitor (PI), and anti-CD38 antibody. In the pivotal, phase 2, single-arm KarMMa trial, ide-cel showed frequent, deep, and durable responses in pts with RRMM who were triple-class exposed to immunomodulatory drugs, PIs and anti-CD38 antibodies (Munshi NC, et al. NEJM 2021;384:705-716). To understand the pts' perspective on the advantages and disadvantages of ide-cel treatment that may not be captured by established health-related quality of life (HRQoL) questionnaires, a series of qualitative pt interviews were embedded into the KarMMa trial. This analysis focuses on time points between 6 and 24 months following treatment with ide-cel, providing an update on previously reported pt perspectives (Braverman J, et al. J Clin Oncol 2020;38(suppl 29). Abstract 155; Braverman J, et al. Value Health 2021;24(suppl 1). Abstract S61).
Methods: Pts enrolled in the KarMMa trial (NCT03361748) were invited to participate in a series of optional interviews conducted at baseline and throughout the follow-up period. We present the results of qualitative interviews from months 6, 9, 12, 18, and 24 post treatment. Interviews were audio-recorded and transcribed. Non-English interviews were transcribed directly into English. Transcripts were analyzed using MAXQDA qualitative analysis software; inter-coder agreement exercises were used to ensure sufficient reliability. Analytic approaches included thematic analysis, quantitative descriptive analysis of close-ended items, and longitudinal analysis across HRQoL domains.
Results: In total, 131 transcripts were analyzed for 45/128 unique pts treated with ide-cel in the US and Europe. The number of pts completing each interview steadily declined, from 36 at month 6 to 19 at month 24. The mean age of pts at the time of giving their consent in the trial was 63.5 years and 44% were female.
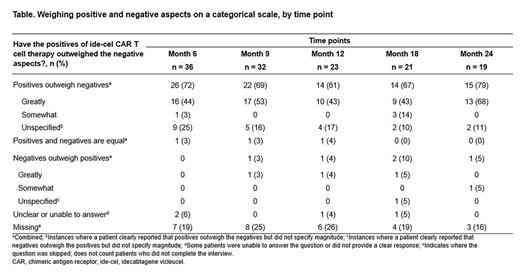
Across time points, most pts (61%-79%) reported that positives of ide-cel outweighed negatives (Table). Overall, few pts reported that the negatives and positives were equal (n = 3), or that negatives outweighed positives (n = 5) across time points. The most frequent advantages were durability of treatment response (n = 23, 51%), improvements in HRQoL (n = 23, 51%), living a "normal life" (n = 19, 42%), and living for longer (n = 13, 29%). Additional frequent advantages were side effects after infusion, described as minor or not at all (n = 35, 78%), and ide-cel being a "one-time" treatment (n = 21, 47%). Common themes related to disadvantages were side effects after infusion (n = 27, 60%), the short response to treatment (n = 23, 51%), durability (n = 16, 36%), and lingering side effects (n = 18, 40%) that continued ≥ 3 months after infusion. Many pts also reported trial-related disadvantages (n = 31, 69%). At months 6, 9, 12, 18, and 24 post treatment, most pts (n = 33, 92%; n = 28, 88%; n = 18, 78%, n = 18, 86%; n = 18, 95%, respectively) reported they would make the same decision to receive ide-cel again, mostly due to positive results they had following treatment. Most pts (n = 29 [81%)], n = 27 [84%], n = 15 [65%], n = 17 [81%], and n = 16 [84%] at months 6, 9, 12, 18, and 24, respectively) reported they would recommend ide-cel treatment to others.
The majority of pts (n = 26, 72%) described improvements in their physical health at 6 months post infusion versus baseline although this worsened for some at months 9-12 (n = 9, 25%) and 18-24 (n = 13, 48%) versus previous time points. Improvement in the emotional functioning domain was less common (n = 13, 36% at month 6 versus baseline, n = 5, 14% at months 9-12 and n = 4, 15% at months 18-24 versus previous time points) with most pts experiencing no change. Being optimistic was a common emotional concept expressed in interviews (n = 33, 73%). About half of pts (n = 21, 47%) experienced anxiety due to worry and fear of potential relapse.
Conclusions: This study provides unique qualitative insights into the pt experience in the 24 months following ide-cel infusion. Overall, pts reported positive treatment experiences throughout interviews. Most pts reported they would make the same decision again to receive ide-cel and would recommend it to others.
Shah: Oncopeptides: Consultancy; Karyopharm: Consultancy; Poseida: Research Funding; Precision Biosciences: Research Funding; Sanofi: Consultancy; Sutro Biopharma: Research Funding; Kite: Consultancy; Janssen: Research Funding; Indapta Therapeutics: Consultancy; Bluebird Bio: Research Funding; BMS/Celgene: Research Funding; GSK: Consultancy; CSL Behring: Consultancy; CareDx: Consultancy; Nektar: Research Funding; Amgen: Consultancy; Teneobio: Research Funding. Delforge: Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Rodríguez-Otero: BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen, Celgene, Amgen, Oncopeptides, Sanofi, Abbvie, GlaxoSmithKline, Kite Pharma: Consultancy, Honoraria, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy; Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Regeneron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Moshkovich: ICON plc: Current Employment. Braverman: BMS: Current Employment, Current equity holder in publicly-traded company. Dhanda: BMS: Current Employment, Current equity holder in publicly-traded company. Lanar: ICON plc: Current Employment; BMS: Consultancy; Roche: Consultancy. Miera: ICON Clinical Research: Current Employment. Gerould: ICON: Current Employment. Campbell: Bristol Myers Squibb: Current Employment, Current holder of individual stocks in a privately-held company. Munshi: Novartis: Consultancy; Abbvie: Consultancy; Bristol-Myers Squibb: Consultancy; Janssen: Consultancy; Karyopharm: Consultancy; Adaptive Biotechnology: Consultancy; Celgene: Consultancy; Takeda: Consultancy; Amgen: Consultancy; Oncopep: Consultancy, Current equity holder in publicly-traded company, Other: scientific founder, Patents & Royalties; Pfizer: Consultancy; Legend: Consultancy.